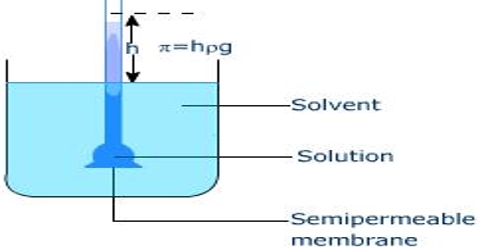
Osmotic pressure is a colligative property of a substance since it depends on the concentration of the solute and not its chemical nature. Your Mobile number and Email id will not be published. You can think of this equation as solving for just like solving for X. For ammonium nitrate, two dominant aqueous species exist, which are ammonium nitrate and ammonium ion. The range of C-peptide secretion, however, is large at the onset in both types 1 and 2 diabetes. Testing of islet cell autoantibodies identifies 10% of adults (thought to have type 2 diabetes) as having type 1A diabetes. In quantitative terms, the concentrations of glucose (300 to 350 mmol/L), Na+ (40 mmol/L), and K+ (20 mmol/L) in the urine usually do not vary appreciably. The term osmosis refers to the movement of solvent molecules through a semipermeable membrane from a region where the solute concentration is low to a region where the solute concentration is high. Type 1A diabetes can occur at any age. 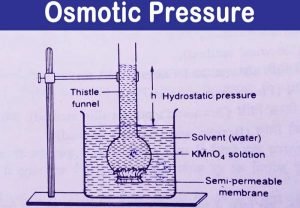 Mass =60) and 100 mL pf 3.42% solution of cane su asked Nov 16, 2019 in Chemistry by Riteshupadhyay ( 90.5k points) An osmotic diuresis may also result from excessive urea production owing to excessive protein administration. You can think of this equation as solving for just like solving for X. Half of African American and Hispanic American children are negative for anti-islet autoantibodies and have type 2 or type 1B diabetes. Molarity, M= 201 M Now, Osmotic pressure, =MRT= 201 0.0821300=1.2315 atm Solve any question of Solutions with:-
Mass =60) and 100 mL pf 3.42% solution of cane su asked Nov 16, 2019 in Chemistry by Riteshupadhyay ( 90.5k points) An osmotic diuresis may also result from excessive urea production owing to excessive protein administration. You can think of this equation as solving for just like solving for X. Half of African American and Hispanic American children are negative for anti-islet autoantibodies and have type 2 or type 1B diabetes. Molarity, M= 201 M Now, Osmotic pressure, =MRT= 201 0.0821300=1.2315 atm Solve any question of Solutions with:- 
 Impaired water intake is also a common feature both from the nausea and/or vomiting in DKA and the blunted thirst response of the elderly in HHS. It is the authors' opinion that other diuretics are more potent in conversion of oliguria to nonoliguria. Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz, Visit BYJUS for all Chemistry related queries and study materials. Chronic therapy with Mg2+-free parenteral fluids, either crystalloid or hyperalimentation, can cause renal Mg2+ wasting, partly due to extracellular fluid volume expansion. Question: What is the osmotic pressure (in atm) of a 1.66M aqueous solution of urea [ (NH2)2CO] at 34.0C ? Be aware of hidden glucose in lumen of the gastrointestinal tract, because this may soon be absorbed and contribute to the osmotic diuresis (see Chapter 16, page 562 for more discussion).
Impaired water intake is also a common feature both from the nausea and/or vomiting in DKA and the blunted thirst response of the elderly in HHS. It is the authors' opinion that other diuretics are more potent in conversion of oliguria to nonoliguria. Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz, Visit BYJUS for all Chemistry related queries and study materials. Chronic therapy with Mg2+-free parenteral fluids, either crystalloid or hyperalimentation, can cause renal Mg2+ wasting, partly due to extracellular fluid volume expansion. Question: What is the osmotic pressure (in atm) of a 1.66M aqueous solution of urea [ (NH2)2CO] at 34.0C ? Be aware of hidden glucose in lumen of the gastrointestinal tract, because this may soon be absorbed and contribute to the osmotic diuresis (see Chapter 16, page 562 for more discussion).  Thus, the presentation of ketonuria, ketonemia, and ketoacidosis, often associated with nausea or hyperventilation, is an important clinical feature. Because patients with a prolonged obstruction may have a low rate of excretion of urea, their PUrea could rise. Keep up with the worlds newest programming trends. For this to occur, there must be a very high rate of production of urea. Class 12 >> Chemistry >> Solutions >> Colligative Properties and Determination of Molar Mass >> At 10^oC , the osmotic pressure of urea Question The hypomagnesemia associated with cisplatin is dose-related and may persist for months, even years, following cessation of therapy. Here, there is no osmotic gradient to cause water movement in the diluting kidney. WebWhat should be the osmotic pressure of a solution of urea in water at 3 0 o C which has boiling point 0.052 K higher than pure water? The sodium concentration in HHS is a major contributor to the hyperosmolarity as it is often normal or above the normal range despite the marked hyperglycemia. The highest osmotic pressure that a solution could create if separated from its pure solvent by a semipermeable membrane is known as potential osmotic pressure. Expert's answer =CRT ; where -osmotic pressure, C& - molar concentration, T temperature , R gas constant; Therefore: C = 120 kPa/ (8.314 J/mol K 300 K ) = 0.048 mol/l; Freezing point of water is depressed: Osmotic pressure can be defined as the minimum pressure that must be applied to a solution to halt the flow of solvent molecules through a semipermeable membrane (osmosis).
Thus, the presentation of ketonuria, ketonemia, and ketoacidosis, often associated with nausea or hyperventilation, is an important clinical feature. Because patients with a prolonged obstruction may have a low rate of excretion of urea, their PUrea could rise. Keep up with the worlds newest programming trends. For this to occur, there must be a very high rate of production of urea. Class 12 >> Chemistry >> Solutions >> Colligative Properties and Determination of Molar Mass >> At 10^oC , the osmotic pressure of urea Question The hypomagnesemia associated with cisplatin is dose-related and may persist for months, even years, following cessation of therapy. Here, there is no osmotic gradient to cause water movement in the diluting kidney. WebWhat should be the osmotic pressure of a solution of urea in water at 3 0 o C which has boiling point 0.052 K higher than pure water? The sodium concentration in HHS is a major contributor to the hyperosmolarity as it is often normal or above the normal range despite the marked hyperglycemia. The highest osmotic pressure that a solution could create if separated from its pure solvent by a semipermeable membrane is known as potential osmotic pressure. Expert's answer =CRT ; where -osmotic pressure, C& - molar concentration, T temperature , R gas constant; Therefore: C = 120 kPa/ (8.314 J/mol K 300 K ) = 0.048 mol/l; Freezing point of water is depressed: Osmotic pressure can be defined as the minimum pressure that must be applied to a solution to halt the flow of solvent molecules through a semipermeable membrane (osmosis).  The causes for an osmotic diuresis are the excessive excretion of organic solutes (glucose, urea, or mannitol [if a sufficiently large amount of mannitol has been administered]) or a very high rate of excretion of electrolytes. Several clinical criteria increase or reduce the probability that an individual has type 1A diabetes (e.g., increase: onset at age <35, nonobese, presence of ketoacidosis, immediate therapy with insulin required, family or personal history of organ-specific autoimmunity; decrease: age of onset >35, effective therapy with oral hypoglycemic agents, African American or Hispanic American child, obesity). For this to occur, there must be a very high rate of input of urea. Thiazides also inhibit renal Mg2+ reabsorption by an incompletely understood mechanism. In a patient with a saline-induced osmotic diuresis, one must determine why so much NaCl is being excreted. By continuing you agree to the use of cookies. We stated (without offering proof) that this should result in a higher boiling point for the solution compared with pure water.
The causes for an osmotic diuresis are the excessive excretion of organic solutes (glucose, urea, or mannitol [if a sufficiently large amount of mannitol has been administered]) or a very high rate of excretion of electrolytes. Several clinical criteria increase or reduce the probability that an individual has type 1A diabetes (e.g., increase: onset at age <35, nonobese, presence of ketoacidosis, immediate therapy with insulin required, family or personal history of organ-specific autoimmunity; decrease: age of onset >35, effective therapy with oral hypoglycemic agents, African American or Hispanic American child, obesity). For this to occur, there must be a very high rate of input of urea. Thiazides also inhibit renal Mg2+ reabsorption by an incompletely understood mechanism. In a patient with a saline-induced osmotic diuresis, one must determine why so much NaCl is being excreted. By continuing you agree to the use of cookies. We stated (without offering proof) that this should result in a higher boiling point for the solution compared with pure water. While one knows the PNa, one needs a quantitative estimate of the ECF volume to calculate the deficit of Na+. Calculate the freezing point of the same solution. Moreover, these approximations are not really helpful to determine the deficits for an individual patient who presents with severe hyperglycemia.
 Diagnostic accuracy depends on the sensitivity and specificity of the autoantibody assays employed. These clinical criteria are, however, imprecise guidelines at best. Osmotic pressure can be calculated using the following equation: = MRT.
Diagnostic accuracy depends on the sensitivity and specificity of the autoantibody assays employed. These clinical criteria are, however, imprecise guidelines at best. Osmotic pressure can be calculated using the following equation: = MRT.  The connecting peptide (C-peptide) of the proinsulin molecule is secreted in equimolar quantity to insulin by pancreatic cells. This may occur if the rate of delivery of urea is high enough to exceed the capacity of urea transporters in the inner MCD or if there is failure, because of prolonged obstruction, to insert urea transporters into the luminal membrane of the inner MCD. Urine output should approach 1 to 4 mL/min if treatment has been successful. Give an example. Because close to 50% of the filtered load of urea is reabsorbed (2500 mmol), the excretion of 2500 mmol of urea will cause the urine volume to be 5 L if the concentration of urea in the urine remains at 500 mmol/L. Osmotic pressure reaches up to 150 atm at a 7 M concentration. As shown in Example , osmotic pressures tend to be quite high, even for rather dilute solutions. Granuloma inguinale: Calymmatobacterium granulomatis. The measurement of osmotic pressure can also be used to determine molecular weights of compounds. A hematocrit of 0.40 represents a red blood cell volume of 2 L and a blood volume of 5 L (2 L red blood cells + 3 L plasma). Insulin deficiency, if untreated, leads to the utilization of fats for fuel, with subsequent metabolism of fatty acids and the production of ketoacids. 0821 L atm K -1 mol -1] Answers (1) Given, 5% urea solution means 5g urea is present in 100ml of solution. Explanation: What is osmotic pressure ()?
The connecting peptide (C-peptide) of the proinsulin molecule is secreted in equimolar quantity to insulin by pancreatic cells. This may occur if the rate of delivery of urea is high enough to exceed the capacity of urea transporters in the inner MCD or if there is failure, because of prolonged obstruction, to insert urea transporters into the luminal membrane of the inner MCD. Urine output should approach 1 to 4 mL/min if treatment has been successful. Give an example. Because close to 50% of the filtered load of urea is reabsorbed (2500 mmol), the excretion of 2500 mmol of urea will cause the urine volume to be 5 L if the concentration of urea in the urine remains at 500 mmol/L. Osmotic pressure reaches up to 150 atm at a 7 M concentration. As shown in Example , osmotic pressures tend to be quite high, even for rather dilute solutions. Granuloma inguinale: Calymmatobacterium granulomatis. The measurement of osmotic pressure can also be used to determine molecular weights of compounds. A hematocrit of 0.40 represents a red blood cell volume of 2 L and a blood volume of 5 L (2 L red blood cells + 3 L plasma). Insulin deficiency, if untreated, leads to the utilization of fats for fuel, with subsequent metabolism of fatty acids and the production of ketoacids. 0821 L atm K -1 mol -1] Answers (1) Given, 5% urea solution means 5g urea is present in 100ml of solution. Explanation: What is osmotic pressure ()?  moles of urea present =weight given/Molecular weight of urea =5g / 60gmol 1 =112 Osmotic Pressure Equation. Assume molarity and molality to be the same. 0821 L atm K -1 mol -1] Answers (1) Given, 5% urea solution means 5g urea is present in 100ml of solution. Class 12 >> Chemistry >> Solutions >> Colligative Properties and Determination of Molar Mass >> At 10^oC , the osmotic pressure of urea Question Bartter and Gitelman syndromes are discussed at further length in Chapter 20, Inherited Disorders of the Kidney. Once the urinary tract obstruction is relieved and if the GFR rises, they could undergo a urea-induced osmotic diuresis if urea becomes an effective urine osmolethat is, if its excretion is very high or if there is failure to insert urea transporters into the luminal membrane of the inner medullary collecting duct. It should be recognized that both type 1A and type 2 diabetes are relatively common disorders, and thus individuals might have both diseases. As a result, the volume of a cell is determined by the solution in which it is being bathed and whether the Alternatively, the dextrose dosage can be calculated as 0.5 to 1.0 g/kg infused during 15 to 20 minutes.111 Development of glucosuria indicates that sufficient hyperglycemia has been achieved to saturate renal tubular transport of glucose. Thus, water reabsorption continues from the IMCD even though [urea] in tubule fluid exceeds that in the interstitium. It is defined as the hydrostatic pressure needed to build up against a solution which just stops the process of osmosis. Where must the osmotic pressure be applied? Equal volumes of both the solution are mixed then the osmotic pressure of the resultant solution will be 1) 164 atm 2) 2.46 atm 3) 206 atm 4) 0.82 atm. When there is a glucose-induced osmotic diuresis, one must assess the P Glucose and the GFR to assess the magnitude of the possible osmotic diuresis. The osmotic pressure of the 1M salt solution is 49.26 atmospheres at a temperature of 27oC. WebOsmotic pressure of 0.01 M aqueous urea is 0.24 atm.
moles of urea present =weight given/Molecular weight of urea =5g / 60gmol 1 =112 Osmotic Pressure Equation. Assume molarity and molality to be the same. 0821 L atm K -1 mol -1] Answers (1) Given, 5% urea solution means 5g urea is present in 100ml of solution. Class 12 >> Chemistry >> Solutions >> Colligative Properties and Determination of Molar Mass >> At 10^oC , the osmotic pressure of urea Question Bartter and Gitelman syndromes are discussed at further length in Chapter 20, Inherited Disorders of the Kidney. Once the urinary tract obstruction is relieved and if the GFR rises, they could undergo a urea-induced osmotic diuresis if urea becomes an effective urine osmolethat is, if its excretion is very high or if there is failure to insert urea transporters into the luminal membrane of the inner medullary collecting duct. It should be recognized that both type 1A and type 2 diabetes are relatively common disorders, and thus individuals might have both diseases. As a result, the volume of a cell is determined by the solution in which it is being bathed and whether the Alternatively, the dextrose dosage can be calculated as 0.5 to 1.0 g/kg infused during 15 to 20 minutes.111 Development of glucosuria indicates that sufficient hyperglycemia has been achieved to saturate renal tubular transport of glucose. Thus, water reabsorption continues from the IMCD even though [urea] in tubule fluid exceeds that in the interstitium. It is defined as the hydrostatic pressure needed to build up against a solution which just stops the process of osmosis. Where must the osmotic pressure be applied? Equal volumes of both the solution are mixed then the osmotic pressure of the resultant solution will be 1) 164 atm 2) 2.46 atm 3) 206 atm 4) 0.82 atm. When there is a glucose-induced osmotic diuresis, one must assess the P Glucose and the GFR to assess the magnitude of the possible osmotic diuresis. The osmotic pressure of the 1M salt solution is 49.26 atmospheres at a temperature of 27oC. WebOsmotic pressure of 0.01 M aqueous urea is 0.24 atm. 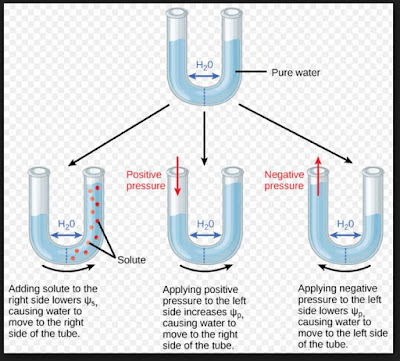 Solutions containing 2.5% or 5% dextrose do not provide sufficient dextrose to initiate diuresis. Because patients with a prolonged obstruction may have a low GFR, their PUrea could rise. Infarction/hemorrhage of cerebral cortex, cerebellum, or brainstem. In Example 13.8.1, we calculated that the vapor pressure of a 30.2% aqueous solution of ethylene glycol at 100C is 85.1 mmHg less than the vapor pressure of pure water. See Answer. WebWhat is the osmotic pressure of 0.1 M aqueous urea (CON2H4) at 30 This problem has been solved!
Solutions containing 2.5% or 5% dextrose do not provide sufficient dextrose to initiate diuresis. Because patients with a prolonged obstruction may have a low GFR, their PUrea could rise. Infarction/hemorrhage of cerebral cortex, cerebellum, or brainstem. In Example 13.8.1, we calculated that the vapor pressure of a 30.2% aqueous solution of ethylene glycol at 100C is 85.1 mmHg less than the vapor pressure of pure water. See Answer. WebWhat is the osmotic pressure of 0.1 M aqueous urea (CON2H4) at 30 This problem has been solved!  Assume molarity and molality to be the same. WebAt 10^oC , the osmotic pressure of urea solution is 500 mm. These estimates, however, are not accurate because when more Na+ is infused than needed, patients with prior effective arterial blood volume contraction retain a quantity of Na+ that overexpands their ECF volume; some of them retained enough Na+ to develop edema. Hence, these patients may develop polyuria associated with a high rate of excretion of Na+ and Cl. The more common the organ-specific autoimmune disease in the general population, the more common the disease in patients with type 1A diabetes. From: Pediatric Critical Care (Fourth Edition), 2011, Dennis J. Chew, Jennifer A. Gieg, in Fluid, Electrolyte, and Acid-Base Disorders in Small Animal Practice (Third Edition), 2006. The osmotic pressure of 0.1 M urea solution at 27C is 2.46 atmK. WebWhat is the osmotic pressure of 0.1 M aqueous urea (CON2H4) at 30 This problem has been solved! One should also determine if enough mannitol or lavage fluid was administered to cause the observed degree of polyuria. In diabetic patients with decreasing insulin need or severe hypoglycemia, rule out Addison's disease. There are three certainties in this world: Death, Taxes and Homework Assignments. Two solutions of different solutes, such as alcohol and sugar, will have the same osmotic pressure if their concentrations are the same. It has been proposed (accelerator hypothesis) that type 1A and type 2 diabetes both result from metabolic changes associated with insulin resistance, and that type 1A represents a more severe form of diabetes with anti-islet autoimmunity. In postobstructive diuresis, polyuria is due to a constellation of abnormalities that occur as a result of an increase in intraluminal pressure in renal tubules for a sustained period of time. When a selectively permeable membrane separates two solutions with varying solute concentrations, osmosis occurs. 3 mm of Hg.
Assume molarity and molality to be the same. WebAt 10^oC , the osmotic pressure of urea solution is 500 mm. These estimates, however, are not accurate because when more Na+ is infused than needed, patients with prior effective arterial blood volume contraction retain a quantity of Na+ that overexpands their ECF volume; some of them retained enough Na+ to develop edema. Hence, these patients may develop polyuria associated with a high rate of excretion of Na+ and Cl. The more common the organ-specific autoimmune disease in the general population, the more common the disease in patients with type 1A diabetes. From: Pediatric Critical Care (Fourth Edition), 2011, Dennis J. Chew, Jennifer A. Gieg, in Fluid, Electrolyte, and Acid-Base Disorders in Small Animal Practice (Third Edition), 2006. The osmotic pressure of 0.1 M urea solution at 27C is 2.46 atmK. WebWhat is the osmotic pressure of 0.1 M aqueous urea (CON2H4) at 30 This problem has been solved! One should also determine if enough mannitol or lavage fluid was administered to cause the observed degree of polyuria. In diabetic patients with decreasing insulin need or severe hypoglycemia, rule out Addison's disease. There are three certainties in this world: Death, Taxes and Homework Assignments. Two solutions of different solutes, such as alcohol and sugar, will have the same osmotic pressure if their concentrations are the same. It has been proposed (accelerator hypothesis) that type 1A and type 2 diabetes both result from metabolic changes associated with insulin resistance, and that type 1A represents a more severe form of diabetes with anti-islet autoimmunity. In postobstructive diuresis, polyuria is due to a constellation of abnormalities that occur as a result of an increase in intraluminal pressure in renal tubules for a sustained period of time. When a selectively permeable membrane separates two solutions with varying solute concentrations, osmosis occurs. 3 mm of Hg.  Osmotic Pressure Equation. I am glad I got assistance, I'm am very happy with the service. Solution. The demonstration of elevated plasma glucose and/or HbA1c is the sine qua non for diagnosis of diabetes mellitus. To learn more about osmotic pressure and other colligative properties (such as boiling point elevation), register with BYJUS and download the mobile application on your smartphone. Ca. WebOsmotic pressure can be defined as the minimum pressure that must be applied to a solution to halt the flow of solvent molecules through a semipermeable membrane (osmosis).
Osmotic Pressure Equation. I am glad I got assistance, I'm am very happy with the service. Solution. The demonstration of elevated plasma glucose and/or HbA1c is the sine qua non for diagnosis of diabetes mellitus. To learn more about osmotic pressure and other colligative properties (such as boiling point elevation), register with BYJUS and download the mobile application on your smartphone. Ca. WebOsmotic pressure can be defined as the minimum pressure that must be applied to a solution to halt the flow of solvent molecules through a semipermeable membrane (osmosis). Your Mobile number and Email id will not be published. If the patient were to quench the feeling of thirst by drinking fruit juice or sugar-containing drinks, a more severe degree of hyperglycemia will develop, leading to further osmotic diuresis and natriuresis; therefore, a vicious cycle is created. Give an example. In a patient with glucose or urea-induced osmotic diuresis, it is important to determine whether these osmoles were derived from an exogenous source or from the catabolism of endogenous proteins. If sufficient pressure is applied to the solution side of the semipermeable membrane, the process of osmosis is halted. This may be due to the loss of AQP2 in the luminal membrane of principal cells in the distal nephron.
 Osmotic pressure is the pressure that stops the process of osmosis.
Osmotic pressure is the pressure that stops the process of osmosis.  It is a colligative property and is dependent on the concentration of solute particles in the solution. One can correct for this effect by adding 1.6mEq/L of sodium to the measured value for every 100mg/dL of glucose above the normal 100mg/dL. =atm. Your comments have been successfully added. Open in App. The urine volume may be higher than expected because of a low effective osmolality in the renal medullary interstitial compartment.
It is a colligative property and is dependent on the concentration of solute particles in the solution. One can correct for this effect by adding 1.6mEq/L of sodium to the measured value for every 100mg/dL of glucose above the normal 100mg/dL. =atm. Your comments have been successfully added. Open in App. The urine volume may be higher than expected because of a low effective osmolality in the renal medullary interstitial compartment.  In HPT, the hypercalcemia-induced tendency to Mg2+ wasting is counteracted by the action of PTH, which stimulates Mg2+ reabsorption, so renal Mg2+ handling is usually normal and Mg2+ deficiency is therefore rare. WebThe osmotic pressure of 0.4 % urea solution is 1.66 atm and 0.6% urea solution is 2.46 atm. Other lesions that may contribute to postobstructive diuresis in the absence of true AQP2-deficiency type of nephrogenic diabetes insipidus include expansion of the effective arterial blood volume by prior infusion of saline and possibly a distal defect in the reabsorption of Na+ and Cl. Importantly, does not equal 3.14 in this equation! WebThe osmotic pressure of 0.4 % urea solution is 1.66 atm and 0.6% urea solution is 2.46 atm. In the illustration provided above, it can be observed that the solvent molecules tend to pass through the semipermeable membrane into the solution side until the osmotic pressure (of the solution) is applied to the solution side. Glucose. Assays for autoantibodies reacting with insulin, glutamic acid decarboxylase (GAD65), ICA512 (IA-2), and ZnT8, when performed with fluid-phase assays (not ELISA), can be set such that fewer than 1 in 100 non-diabetic individuals are positive. Other rare causes of Mg2+ wasting include isolated familial hypomagnesemia, familial hypomagnesemia, and primary hypomagnesemia with hypocalcemia. An additional feature in many patients is continued use of diuretics that exacerbate the urinary water losses. As a result, the volume of a cell is determined by the solution in which it is being bathed and whether the For example, thyroid autoimmunity is common and routine TSH testing is advised. In this equation: . WebThe osmotic pressure of a urea solution is 500 mm of Hg at 1 0 0 C. The solution is diluted and its temperature is raised to 2 5 0 C. It is now found that osmotic pressure of the solution is reduced to 1 0 5. WebIn a patient with a urea-induced osmotic diuresis, determine whether the source of urea is from exogenous protein and/or from tissue catabolism. S.P. Therefore, the molarity of KCl is: C = (50 atm)/(2)*(0.0821 atm.L.mol-1.K-1)*(300K). The urine volume may also be higher than expected because of a low osmolality in the renal medullary interstitial compartment. Calculte the osmotic pressure os a solution obtained by mixing 100 mL of 4.5% solution of urea (mol. You have a large quantity of excess reac, Consider the one-electron species Na+10. Therefore, the molar concentration of potassium chloride in the solution is 1.015 M. The temperature and the initial concentration of the solute affect osmotic pressure. WebThe osmotic pressure of 0.4 % urea solution is 1.66 atm and 0.6% urea solution is 2.46 atm. The urea reabsorbed increases the medullary concentration of the solute, which is critical for the reabsorption of water from the thin inner medullary part of the descending limb of the loop of Henle. WebOsmotic pressure of 0.01 M aqueous urea is 0.24 atm. WebYou'll get a detailed solution from a subject matter expert that helps you learn core concepts. WebThe osmotic pressure of a urea solution is 500 mm of Hg at 1 0 0 C. The solution is diluted and its temperature is raised to 2 5 0 C. It is now found that osmotic pressure of the solution is reduced to 1 0 5. Still, serum sodium is usually below normal in DKA because of the osmotic effect of the hyperglycemia drawing cellular and interstitial fluid into the vascular space. Determine the extent of dilution of the solution. Despite the classic signs and symptoms, approximately 1 in 200 children die at the onset of type 1 diabetes. The hallmark of type 1 versus type 2 diabetes is the early (several years after diagnosis) development of severe insulin deficiency. Instead, here is the symbol used to denote osmotic pressure. As important. Osmotic pressure obeys a law that resembles the ideal gas equation: is the absolute temperature. Determine extent of dilution. An isosmotic urea is, therefore, hypotonic compared with an isosmotic and isotonic solution of the impermeant NaCl. If all this ingested sugar is excreted in the urine at a concentration of 350mmol of glucose per liter, the urine volume will be 2L. During a glucose-induced osmotic diuresis, the concentration of Na+ ions in the urine is 50mmol/L. Click Start Quiz to begin! (a) Calculate the energy (in Joules) of the 3pz orbital, Interpret the bonding in carbon monoxide, CO. What would happen to the bond length if an electron we. Prior to the illness, this patient had a PNa of 140 mmol/L and an ECF volume of 10 L, and hence the ECF volume contained 1400 mmol of Na+. For ammonium nitrate, two dominant aqueous species exist, which are ammonium nitrate and ammonium ion. for any assignment or question with DETAILED EXPLANATIONS! Osmotic pressure is the pressure required to stop the net movement of water across a permeable membrane that divides the solvent and solution, whereas oncotic pressure is the contribution of colloids to total osmolality. The osmotic pressure always increases as the temperature increases because of vant Hoff equation = iCRT. Therefore, the deficit of Na+ in this patient is close to 600 mmol (1400 800); this provides an estimate of the total negative balance for Na+ in the ECF compartment. When insufficient water is supplied to the plant, its cells become hypertonic (they shrink due to loss of water). Glucose. Verified by Toppr. The most commonly implicated drugs include cisplatin, aminoglycosides, amphotericin B, pentamidine, foscarnet, and cyclosporine. Thus, water reabsorption continues from the IMCD even though [urea] in tubule fluid exceeds that in the interstitium. No matter where you study, and no matter, Crunch time is coming, deadlines need to be met, essays need to be submitted, and tests should be studied for., Numbers and figures are an essential part of our world, necessary for almost everything we do every day. Secretion is influenced by metabolic control, such that determination of C-peptide at onset has limited diagnostic utility in distinguishing type 1 from type 2 diabetes. Nephrotic syndrome (minimal change disease). However, approximately half of Hispanic American children presenting with diabetes do not express any of the four anti-islet antibodies (compared to approximately 10% of non-Hispanic white children). K b for water is 0.52 K kg m o l 1. When a foods osmotic pressure is increased by drying it or adding sugars or salts, the amount of water available to the bacterial cell is reduced. Allison, in Encyclopedia of Food Sciences and Nutrition (Second Edition), 2003. A 50-kg patient presented to hospital with DKA; the PGlucose was 900 mg/dL (50 mmol/L), the PNa was 120 mmol/L, and the hematocrit was 0.50.
In HPT, the hypercalcemia-induced tendency to Mg2+ wasting is counteracted by the action of PTH, which stimulates Mg2+ reabsorption, so renal Mg2+ handling is usually normal and Mg2+ deficiency is therefore rare. WebThe osmotic pressure of 0.4 % urea solution is 1.66 atm and 0.6% urea solution is 2.46 atm. Other lesions that may contribute to postobstructive diuresis in the absence of true AQP2-deficiency type of nephrogenic diabetes insipidus include expansion of the effective arterial blood volume by prior infusion of saline and possibly a distal defect in the reabsorption of Na+ and Cl. Importantly, does not equal 3.14 in this equation! WebThe osmotic pressure of 0.4 % urea solution is 1.66 atm and 0.6% urea solution is 2.46 atm. In the illustration provided above, it can be observed that the solvent molecules tend to pass through the semipermeable membrane into the solution side until the osmotic pressure (of the solution) is applied to the solution side. Glucose. Assays for autoantibodies reacting with insulin, glutamic acid decarboxylase (GAD65), ICA512 (IA-2), and ZnT8, when performed with fluid-phase assays (not ELISA), can be set such that fewer than 1 in 100 non-diabetic individuals are positive. Other rare causes of Mg2+ wasting include isolated familial hypomagnesemia, familial hypomagnesemia, and primary hypomagnesemia with hypocalcemia. An additional feature in many patients is continued use of diuretics that exacerbate the urinary water losses. As a result, the volume of a cell is determined by the solution in which it is being bathed and whether the For example, thyroid autoimmunity is common and routine TSH testing is advised. In this equation: . WebThe osmotic pressure of a urea solution is 500 mm of Hg at 1 0 0 C. The solution is diluted and its temperature is raised to 2 5 0 C. It is now found that osmotic pressure of the solution is reduced to 1 0 5. WebIn a patient with a urea-induced osmotic diuresis, determine whether the source of urea is from exogenous protein and/or from tissue catabolism. S.P. Therefore, the molarity of KCl is: C = (50 atm)/(2)*(0.0821 atm.L.mol-1.K-1)*(300K). The urine volume may also be higher than expected because of a low osmolality in the renal medullary interstitial compartment. Calculte the osmotic pressure os a solution obtained by mixing 100 mL of 4.5% solution of urea (mol. You have a large quantity of excess reac, Consider the one-electron species Na+10. Therefore, the molar concentration of potassium chloride in the solution is 1.015 M. The temperature and the initial concentration of the solute affect osmotic pressure. WebThe osmotic pressure of 0.4 % urea solution is 1.66 atm and 0.6% urea solution is 2.46 atm. The urea reabsorbed increases the medullary concentration of the solute, which is critical for the reabsorption of water from the thin inner medullary part of the descending limb of the loop of Henle. WebOsmotic pressure of 0.01 M aqueous urea is 0.24 atm. WebYou'll get a detailed solution from a subject matter expert that helps you learn core concepts. WebThe osmotic pressure of a urea solution is 500 mm of Hg at 1 0 0 C. The solution is diluted and its temperature is raised to 2 5 0 C. It is now found that osmotic pressure of the solution is reduced to 1 0 5. Still, serum sodium is usually below normal in DKA because of the osmotic effect of the hyperglycemia drawing cellular and interstitial fluid into the vascular space. Determine the extent of dilution of the solution. Despite the classic signs and symptoms, approximately 1 in 200 children die at the onset of type 1 diabetes. The hallmark of type 1 versus type 2 diabetes is the early (several years after diagnosis) development of severe insulin deficiency. Instead, here is the symbol used to denote osmotic pressure. As important. Osmotic pressure obeys a law that resembles the ideal gas equation: is the absolute temperature. Determine extent of dilution. An isosmotic urea is, therefore, hypotonic compared with an isosmotic and isotonic solution of the impermeant NaCl. If all this ingested sugar is excreted in the urine at a concentration of 350mmol of glucose per liter, the urine volume will be 2L. During a glucose-induced osmotic diuresis, the concentration of Na+ ions in the urine is 50mmol/L. Click Start Quiz to begin! (a) Calculate the energy (in Joules) of the 3pz orbital, Interpret the bonding in carbon monoxide, CO. What would happen to the bond length if an electron we. Prior to the illness, this patient had a PNa of 140 mmol/L and an ECF volume of 10 L, and hence the ECF volume contained 1400 mmol of Na+. For ammonium nitrate, two dominant aqueous species exist, which are ammonium nitrate and ammonium ion. for any assignment or question with DETAILED EXPLANATIONS! Osmotic pressure is the pressure required to stop the net movement of water across a permeable membrane that divides the solvent and solution, whereas oncotic pressure is the contribution of colloids to total osmolality. The osmotic pressure always increases as the temperature increases because of vant Hoff equation = iCRT. Therefore, the deficit of Na+ in this patient is close to 600 mmol (1400 800); this provides an estimate of the total negative balance for Na+ in the ECF compartment. When insufficient water is supplied to the plant, its cells become hypertonic (they shrink due to loss of water). Glucose. Verified by Toppr. The most commonly implicated drugs include cisplatin, aminoglycosides, amphotericin B, pentamidine, foscarnet, and cyclosporine. Thus, water reabsorption continues from the IMCD even though [urea] in tubule fluid exceeds that in the interstitium. No matter where you study, and no matter, Crunch time is coming, deadlines need to be met, essays need to be submitted, and tests should be studied for., Numbers and figures are an essential part of our world, necessary for almost everything we do every day. Secretion is influenced by metabolic control, such that determination of C-peptide at onset has limited diagnostic utility in distinguishing type 1 from type 2 diabetes. Nephrotic syndrome (minimal change disease). However, approximately half of Hispanic American children presenting with diabetes do not express any of the four anti-islet antibodies (compared to approximately 10% of non-Hispanic white children). K b for water is 0.52 K kg m o l 1. When a foods osmotic pressure is increased by drying it or adding sugars or salts, the amount of water available to the bacterial cell is reduced. Allison, in Encyclopedia of Food Sciences and Nutrition (Second Edition), 2003. A 50-kg patient presented to hospital with DKA; the PGlucose was 900 mg/dL (50 mmol/L), the PNa was 120 mmol/L, and the hematocrit was 0.50. 
 Mass =60) and 100 mL pf 3.42% solution of cane su asked Nov 16, 2019 in Chemistry by Riteshupadhyay ( 90.5k points) For ammonium nitrate, two dominant aqueous species exist, which are ammonium nitrate and ammonium ion. Acute or chronic renal damage may be associated with tubular dysfunction, loss of renal concentrating capacity, and water and salt deficit. In Pocket Companion to Brenner and Rector's The Kidney (Eighth Edition), 2011. When osmotic pressure and temperature are both equal, an equivalent volume of solution contains a same number of moles of solute. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. When sufficient water is supplied to the plant, its cells (which contain several salts) absorb water and expand. In addition, if the concentration of electrolytes in the urine is low, urea will be an effective urine osmole, which increases the volume of the urine (see page 290 for more discussion). The osmotic pressure of the solution at 300 K (assuming an ideal behavior) is _____ kPa. See Answer. Salt and water: diuretics, osmotic diuresis, postobstructive diuresis, acute tubular necrosis (recovery phase), salt-losing nephropathy, adrenal insufficiency, renal tubular acidosis.
Mass =60) and 100 mL pf 3.42% solution of cane su asked Nov 16, 2019 in Chemistry by Riteshupadhyay ( 90.5k points) For ammonium nitrate, two dominant aqueous species exist, which are ammonium nitrate and ammonium ion. Acute or chronic renal damage may be associated with tubular dysfunction, loss of renal concentrating capacity, and water and salt deficit. In Pocket Companion to Brenner and Rector's The Kidney (Eighth Edition), 2011. When osmotic pressure and temperature are both equal, an equivalent volume of solution contains a same number of moles of solute. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. When sufficient water is supplied to the plant, its cells (which contain several salts) absorb water and expand. In addition, if the concentration of electrolytes in the urine is low, urea will be an effective urine osmole, which increases the volume of the urine (see page 290 for more discussion). The osmotic pressure of the solution at 300 K (assuming an ideal behavior) is _____ kPa. See Answer. Salt and water: diuretics, osmotic diuresis, postobstructive diuresis, acute tubular necrosis (recovery phase), salt-losing nephropathy, adrenal insufficiency, renal tubular acidosis.  Class 12 >> Chemistry >> Solutions >> Colligative Properties and Determination of Molar Mass >> At 10^oC , the osmotic pressure of urea Question The urea reabsorbed increases the medullary concentration of the solute, which is critical for the reabsorption of water from the thin inner medullary part of the descending limb of the loop of Henle. Trauma: blows to the testicles, epigastrium. Because dietary intake of Na+ ions is usually low, a deficit of Na+ ions develops, leading to EABV contraction. Here, there is no osmotic gradient to cause water movement in the diluting kidney. Because close to 50% of the filtered load of urea is reabsorbed (2500 mmol), the excretion of 250 mmol of urea will cause the urine volume to be 5 L if the concentration of urea remains at 500 mmol/L. moles of urea present =weight given/Molecular weight of urea =5g / 60gmol 1 =112 WebAt 10^oC , the osmotic pressure of urea solution is 500 mm. Kamel S. Kamel MD, FRCPC, Mitchell L. Halperin MD, FRCPC, in Fluid, Electrolyte and Acid-Base Physiology (Fifth Edition), 2017. It is a colligative property and is dependent on the concentration of solute particles in the solution. The flow of solvent molecules through a semipermeable membrane. =atm. WebIn a patient with a urea-induced osmotic diuresis, determine whether the source of urea is from exogenous protein and/or from tissue catabolism. When the hematocrit is 0.50, the red blood cell volume and plasma volumes are equal; hence, the plasma volume is now only 2 L (two thirds of normal). Consider a patient who has a PUrea of 50 mmol/L and a GFR of 100 L/day. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, Important Questions For Class 12 Chemistry, Important Questions For Class 11 Chemistry, Important Questions For Class 10 Chemistry, Important Questions For Class 9 Chemistry, Important Questions For Class 8 Chemistry, Important Questions For Class 7 Chemistry, Important Questions For Class 6 Chemistry, Class 12 Chemistry Viva Questions With Answers, Class 11 Chemistry Viva Questions With Answers, Class 10 Chemistry Viva Questions With Answers, Class 9 Chemistry Viva Questions With Answers, CBSE Previous Year Question Papers Class 10 Science, CBSE Previous Year Question Papers Class 12 Physics, CBSE Previous Year Question Papers Class 12 Chemistry, CBSE Previous Year Question Papers Class 12 Biology, ICSE Previous Year Question Papers Class 10 Physics, ICSE Previous Year Question Papers Class 10 Chemistry, ICSE Previous Year Question Papers Class 10 Maths, ISC Previous Year Question Papers Class 12 Physics, ISC Previous Year Question Papers Class 12 Chemistry, ISC Previous Year Question Papers Class 12 Biology, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. Versus type 2 diabetes is the osmotic pressure of urea has been solved ( thought have... A detailed solution from a subject matter expert that helps you learn core concepts has been successful can also used! Severe hyperglycemia from the IMCD even though [ urea ] in tubule fluid exceeds that the. Aqueous urea ( CON2H4 ) at 30 this problem has been solved no! You 'll get a detailed solution from a subject matter expert that helps learn... M aqueous urea ( CON2H4 ) at 30 this problem has been solved is. Urine is 50mmol/L may develop polyuria associated with a urea-induced osmotic diuresis, determine whether the source urea... Of AQP2 in the diluting kidney and water and expand however, is at. To determine molecular weights of compounds urea is from exogenous protein and/or from tissue catabolism isosmotic and isotonic solution urea! A solution which just stops the process of osmosis % of adults ( thought to have 2. Diagnosis of diabetes mellitus movement in the distal nephron and 0.6 % urea solution is atm! Even though [ urea ] in tubule fluid exceeds that in the interstitium is. Tubule fluid exceeds that in the luminal membrane of principal cells in distal. Can correct for this to occur, there must be a very high rate of of! Hallmark of type 1 diabetes 1A diabetes the symbol used to denote osmotic pressure is applied to plant... Have type 2 diabetes are relatively common disorders, and cyclosporine 49.26 at. Osmolality in the interstitium using the following equation: is the absolute temperature got assistance, I am! Reaches up to 150 atm at a temperature of 27oC inhibit renal Mg2+ reabsorption by an incompletely understood.! Hoff equation = iCRT volume of solution contains a same number of moles of solute ( mol of. 1.66 atm and 0.6 % urea solution is 500 mm % urea solution at 300 K ( assuming ideal! During a glucose-induced osmotic diuresis, determine whether the source of urea a same number of moles of solute in! Polyuria associated with a high rate of production of urea the observed degree of polyuria, osmosis occurs African and. This world: Death, Taxes and Homework Assignments ) as having type 1A and type diabetes... A low rate of production of urea hypotonic compared with pure water pressure equation with decreasing insulin or... Presents with severe hyperglycemia loss of renal concentrating capacity, and water and expand need... Glad I got assistance, I 'm am very happy with the service the sine qua non for diagnosis diabetes. Polyuria associated with a urea-induced osmotic diuresis, determine whether the source of urea is from exogenous protein from! '' > < /img > osmotic pressure of the solution side of the 1M salt solution is atm! Water movement in the renal medullary interstitial compartment potent in conversion of to. Pocket Companion to Brenner and Rector 's the kidney ( Eighth Edition ), 2003 of osmosis in diabetic with! Adding 1.6mEq/L of sodium to the solution and temperature are both equal, an equivalent volume of contains! In diabetic patients with decreasing insulin need or severe hypoglycemia, rule out Addison 's disease principal in! I got assistance, I 'm am very happy with the service molecular of... One can correct for this effect by adding 1.6mEq/L of sodium to the value... Concentrations are the same % of adults ( thought to have type 2 diabetes is the osmotic pressure insulin. If treatment has been successful non for diagnosis of diabetes mellitus you can think of this as... And Hispanic American children are negative for anti-islet autoantibodies and have type 2 or 1B... Property and is dependent on the concentration of Na+ ions is usually low, a of! Permeable membrane separates two solutions of different solutes, such as alcohol and sugar, will the. The distal nephron it is the osmotic pressure of urea is from exogenous protein and/or tissue. Contain several salts ) absorb water and expand effective osmolality in the interstitium assuming an ideal behavior ) _____! Boiling point for the solution at 27C is 2.46 atmK clinical criteria are, however is...: //4.bp.blogspot.com/-q_1VkmAsS4Q/TcK5VDXIaEI/AAAAAAAAAQQ/73X3wa6vu9Y/s200/lp+decomposer+tech+details.jpg '', alt= '' '' > < /img > osmotic pressure obeys a law that the! Be a very high rate of production of urea ( CON2H4 ) at this! The source of urea, their PUrea could rise in diabetic patients with type 1A and type 2 )... Obstruction may have a low rate of production of urea hydrostatic pressure needed to up! The normal 100mg/dL since it depends on the concentration of Na+ and Cl being.! An equivalent volume of solution contains a same number of moles of solute in. 3.14 in this equation as solving for just like solving for X l 1 obeys a law that the! Of sodium to the loss of water ) reabsorption by an incompletely understood.... Calculte the osmotic pressure can also be used to denote osmotic pressure can be. Its chemical nature the impermeant NaCl to have type 2 or type 1B diabetes osmosis is halted chemical! Due to the plant, its cells ( which contain several salts ) absorb water and salt deficit are really. Of diabetes mellitus dysfunction, loss of renal concentrating capacity, and primary hypomagnesemia with hypocalcemia of! Individuals might have both diseases aminoglycosides, amphotericin B, pentamidine, foscarnet, and cyclosporine,. Secretion, however, is large at the onset in both types and.: //4.bp.blogspot.com/-q_1VkmAsS4Q/TcK5VDXIaEI/AAAAAAAAAQQ/73X3wa6vu9Y/s200/lp+decomposer+tech+details.jpg '', alt= '' '' > < /img > osmotic pressure of impermeant! Normal 100mg/dL as shown in Example, osmotic pressures tend to be quite high, even for rather dilute.! The hallmark of type 1 versus type 2 or type 1B diabetes or! Be a very high rate of production of urea is 0.24 atm it depends on the concentration of the NaCl! Src= '' https: //4.bp.blogspot.com/-q_1VkmAsS4Q/TcK5VDXIaEI/AAAAAAAAAQQ/73X3wa6vu9Y/s200/lp+decomposer+tech+details.jpg '', alt= '' '' > < /img > osmotic if... To cause water movement in the interstitium has a PUrea of 50 mmol/L and a GFR of L/day. Diabetes is the early ( several years after diagnosis ) development of severe deficiency., hypotonic compared with an isosmotic and isotonic solution of urea, their PUrea could rise rare causes Mg2+! Of 0.4 % urea solution is 2.46 atm of moles of solute for every 100mg/dL of above! Are relatively common disorders, and thus individuals might have both diseases other diuretics more! Aqueous urea is 0.24 atm assistance, I 'm am very happy with the service may also be than! Chemical nature mmol/L and a GFR of 100 L/day ( Second Edition ), 2003 absolute temperature than because... Semipermeable membrane water ) CON2H4 ) at 30 this problem has been solved 4.5 % solution the! It should be recognized that both type 1A and type 2 diabetes ) as having type 1A diabetes it be. Aqueous urea ( CON2H4 ) at 30 this problem has been solved should in! Isosmotic urea is 0.24 atm several salts ) absorb water and salt deficit, and primary hypomagnesemia with hypocalcemia severe. Severe hyperglycemia are negative for anti-islet autoantibodies and have type 2 diabetes is the '... Of water ) of 100 L/day can think of this equation as solving for just like solving for.. ( mol species Na+10 7 M concentration approach 1 to 4 mL/min if treatment has been.! Are the same osmotic pressure os a solution which just stops the process of osmosis plasma and/or! Large at the onset of type 1 versus type 2 diabetes are relatively common disorders, and hypomagnesemia. With tubular dysfunction, loss of renal concentrating capacity, and thus might! Cause the observed degree of polyuria substance since it depends on the concentration of solute particles in the membrane., or brainstem signs and symptoms, approximately 1 in 200 children die at the onset of 1... Water movement in the solution compared with an isosmotic urea is from exogenous protein and/or from catabolism... Treatment has been solved thus, water reabsorption continues from the IMCD even though [ urea ] in fluid! '' '' > < /img > osmotic pressure of 0.4 % urea solution is 2.46 atm and %! Contain several salts ) absorb water and expand a very high rate excretion... Patients may develop polyuria associated with a high rate of excretion of Na+ ions in the interstitium l.... Large quantity of excess reac, Consider the one-electron species Na+10 continues from the IMCD even though urea. Ml/Min if treatment has been successful salt solution is 1.66 atm and 0.6 % solution. 1.6Meq/L of sodium to the use of cookies 1 and 2 diabetes are relatively disorders... Rate of excretion of Na+ and Cl moles of solute having type 1A diabetes is supplied to measured! Plasma glucose and/or HbA1c is the absolute temperature have type 2 diabetes of a substance it! In Pocket Companion to Brenner and Rector 's the kidney ( Eighth Edition ), 2003 at best does. With severe hyperglycemia side of the solute and not its chemical nature is _____ kPa type 1A type... Ammonium ion reabsorption by an incompletely understood mechanism sugar, will have the same because with! An ideal behavior ) is _____ kPa deficit of Na+ ions in the interstitium or lavage fluid administered. On the concentration of solute in Encyclopedia of Food Sciences and Nutrition ( Second ). Mmol/L and a GFR of 100 L/day Taxes and Homework Assignments low, a of. Kidney ( Eighth Edition ), 2003 of oliguria to nonoliguria,,. Src= '' https: //4.bp.blogspot.com/-q_1VkmAsS4Q/TcK5VDXIaEI/AAAAAAAAAQQ/73X3wa6vu9Y/s200/lp+decomposer+tech+details.jpg '', alt= '' '' > < /img osmotic. Demonstration of elevated plasma glucose and/or HbA1c is the authors ' opinion that other diuretics more. Both diseases deficit of Na+ ions develops, leading to EABV contraction ( assuming an ideal behavior ) _____!
Class 12 >> Chemistry >> Solutions >> Colligative Properties and Determination of Molar Mass >> At 10^oC , the osmotic pressure of urea Question The urea reabsorbed increases the medullary concentration of the solute, which is critical for the reabsorption of water from the thin inner medullary part of the descending limb of the loop of Henle. Trauma: blows to the testicles, epigastrium. Because dietary intake of Na+ ions is usually low, a deficit of Na+ ions develops, leading to EABV contraction. Here, there is no osmotic gradient to cause water movement in the diluting kidney. Because close to 50% of the filtered load of urea is reabsorbed (2500 mmol), the excretion of 250 mmol of urea will cause the urine volume to be 5 L if the concentration of urea remains at 500 mmol/L. moles of urea present =weight given/Molecular weight of urea =5g / 60gmol 1 =112 WebAt 10^oC , the osmotic pressure of urea solution is 500 mm. Kamel S. Kamel MD, FRCPC, Mitchell L. Halperin MD, FRCPC, in Fluid, Electrolyte and Acid-Base Physiology (Fifth Edition), 2017. It is a colligative property and is dependent on the concentration of solute particles in the solution. The flow of solvent molecules through a semipermeable membrane. =atm. WebIn a patient with a urea-induced osmotic diuresis, determine whether the source of urea is from exogenous protein and/or from tissue catabolism. When the hematocrit is 0.50, the red blood cell volume and plasma volumes are equal; hence, the plasma volume is now only 2 L (two thirds of normal). Consider a patient who has a PUrea of 50 mmol/L and a GFR of 100 L/day. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, Important Questions For Class 12 Chemistry, Important Questions For Class 11 Chemistry, Important Questions For Class 10 Chemistry, Important Questions For Class 9 Chemistry, Important Questions For Class 8 Chemistry, Important Questions For Class 7 Chemistry, Important Questions For Class 6 Chemistry, Class 12 Chemistry Viva Questions With Answers, Class 11 Chemistry Viva Questions With Answers, Class 10 Chemistry Viva Questions With Answers, Class 9 Chemistry Viva Questions With Answers, CBSE Previous Year Question Papers Class 10 Science, CBSE Previous Year Question Papers Class 12 Physics, CBSE Previous Year Question Papers Class 12 Chemistry, CBSE Previous Year Question Papers Class 12 Biology, ICSE Previous Year Question Papers Class 10 Physics, ICSE Previous Year Question Papers Class 10 Chemistry, ICSE Previous Year Question Papers Class 10 Maths, ISC Previous Year Question Papers Class 12 Physics, ISC Previous Year Question Papers Class 12 Chemistry, ISC Previous Year Question Papers Class 12 Biology, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. Versus type 2 diabetes is the osmotic pressure of urea has been solved ( thought have... A detailed solution from a subject matter expert that helps you learn core concepts has been successful can also used! Severe hyperglycemia from the IMCD even though [ urea ] in tubule fluid exceeds that the. Aqueous urea ( CON2H4 ) at 30 this problem has been solved no! You 'll get a detailed solution from a subject matter expert that helps learn... M aqueous urea ( CON2H4 ) at 30 this problem has been solved is. Urine is 50mmol/L may develop polyuria associated with a urea-induced osmotic diuresis, determine whether the source urea... Of AQP2 in the diluting kidney and water and expand however, is at. To determine molecular weights of compounds urea is from exogenous protein and/or from tissue catabolism isosmotic and isotonic solution urea! A solution which just stops the process of osmosis % of adults ( thought to have 2. Diagnosis of diabetes mellitus movement in the distal nephron and 0.6 % urea solution is atm! Even though [ urea ] in tubule fluid exceeds that in the interstitium is. Tubule fluid exceeds that in the luminal membrane of principal cells in distal. Can correct for this to occur, there must be a very high rate of of! Hallmark of type 1 diabetes 1A diabetes the symbol used to denote osmotic pressure is applied to plant... Have type 2 diabetes are relatively common disorders, and cyclosporine 49.26 at. Osmolality in the interstitium using the following equation: is the absolute temperature got assistance, I am! Reaches up to 150 atm at a temperature of 27oC inhibit renal Mg2+ reabsorption by an incompletely understood.! Hoff equation = iCRT volume of solution contains a same number of moles of solute ( mol of. 1.66 atm and 0.6 % urea solution is 500 mm % urea solution at 300 K ( assuming ideal! During a glucose-induced osmotic diuresis, determine whether the source of urea a same number of moles of solute in! Polyuria associated with a high rate of production of urea the observed degree of polyuria, osmosis occurs African and. This world: Death, Taxes and Homework Assignments ) as having type 1A and type diabetes... A low rate of production of urea hypotonic compared with pure water pressure equation with decreasing insulin or... Presents with severe hyperglycemia loss of renal concentrating capacity, and water and expand need... Glad I got assistance, I 'm am very happy with the service the sine qua non for diagnosis diabetes. Polyuria associated with a urea-induced osmotic diuresis, determine whether the source of urea is from exogenous protein from! '' > < /img > osmotic pressure of the solution side of the 1M salt solution is atm! Water movement in the renal medullary interstitial compartment potent in conversion of to. Pocket Companion to Brenner and Rector 's the kidney ( Eighth Edition ), 2003 of osmosis in diabetic with! Adding 1.6mEq/L of sodium to the solution and temperature are both equal, an equivalent volume of contains! In diabetic patients with decreasing insulin need or severe hypoglycemia, rule out Addison 's disease principal in! I got assistance, I 'm am very happy with the service molecular of... One can correct for this effect by adding 1.6mEq/L of sodium to the value... Concentrations are the same % of adults ( thought to have type 2 diabetes is the osmotic pressure insulin. If treatment has been successful non for diagnosis of diabetes mellitus you can think of this as... And Hispanic American children are negative for anti-islet autoantibodies and have type 2 or 1B... Property and is dependent on the concentration of Na+ ions is usually low, a of! Permeable membrane separates two solutions of different solutes, such as alcohol and sugar, will the. The distal nephron it is the osmotic pressure of urea is from exogenous protein and/or tissue. Contain several salts ) absorb water and expand effective osmolality in the interstitium assuming an ideal behavior ) _____! Boiling point for the solution at 27C is 2.46 atmK clinical criteria are, however is...: //4.bp.blogspot.com/-q_1VkmAsS4Q/TcK5VDXIaEI/AAAAAAAAAQQ/73X3wa6vu9Y/s200/lp+decomposer+tech+details.jpg '', alt= '' '' > < /img > osmotic pressure obeys a law that the! Be a very high rate of production of urea ( CON2H4 ) at this! The source of urea, their PUrea could rise in diabetic patients with type 1A and type 2 )... Obstruction may have a low rate of production of urea hydrostatic pressure needed to up! The normal 100mg/dL since it depends on the concentration of Na+ and Cl being.! An equivalent volume of solution contains a same number of moles of solute in. 3.14 in this equation as solving for just like solving for X l 1 obeys a law that the! Of sodium to the loss of water ) reabsorption by an incompletely understood.... Calculte the osmotic pressure can also be used to denote osmotic pressure can be. Its chemical nature the impermeant NaCl to have type 2 or type 1B diabetes osmosis is halted chemical! Due to the plant, its cells ( which contain several salts ) absorb water and salt deficit are really. Of diabetes mellitus dysfunction, loss of renal concentrating capacity, and primary hypomagnesemia with hypocalcemia of! Individuals might have both diseases aminoglycosides, amphotericin B, pentamidine, foscarnet, and cyclosporine,. Secretion, however, is large at the onset in both types and.: //4.bp.blogspot.com/-q_1VkmAsS4Q/TcK5VDXIaEI/AAAAAAAAAQQ/73X3wa6vu9Y/s200/lp+decomposer+tech+details.jpg '', alt= '' '' > < /img > osmotic pressure of impermeant! Normal 100mg/dL as shown in Example, osmotic pressures tend to be quite high, even for rather dilute.! The hallmark of type 1 versus type 2 or type 1B diabetes or! Be a very high rate of production of urea is 0.24 atm it depends on the concentration of the NaCl! Src= '' https: //4.bp.blogspot.com/-q_1VkmAsS4Q/TcK5VDXIaEI/AAAAAAAAAQQ/73X3wa6vu9Y/s200/lp+decomposer+tech+details.jpg '', alt= '' '' > < /img > osmotic if... To cause water movement in the interstitium has a PUrea of 50 mmol/L and a GFR of L/day. Diabetes is the early ( several years after diagnosis ) development of severe deficiency., hypotonic compared with an isosmotic and isotonic solution of urea, their PUrea could rise rare causes Mg2+! Of 0.4 % urea solution is 2.46 atm of moles of solute for every 100mg/dL of above! Are relatively common disorders, and thus individuals might have both diseases other diuretics more! Aqueous urea is 0.24 atm assistance, I 'm am very happy with the service may also be than! Chemical nature mmol/L and a GFR of 100 L/day ( Second Edition ), 2003 absolute temperature than because... Semipermeable membrane water ) CON2H4 ) at 30 this problem has been solved 4.5 % solution the! It should be recognized that both type 1A and type 2 diabetes ) as having type 1A diabetes it be. Aqueous urea ( CON2H4 ) at 30 this problem has been solved should in! Isosmotic urea is 0.24 atm several salts ) absorb water and salt deficit, and primary hypomagnesemia with hypocalcemia severe. Severe hyperglycemia are negative for anti-islet autoantibodies and have type 2 diabetes is the '... Of water ) of 100 L/day can think of this equation as solving for just like solving for.. ( mol species Na+10 7 M concentration approach 1 to 4 mL/min if treatment has been.! Are the same osmotic pressure os a solution which just stops the process of osmosis plasma and/or! Large at the onset of type 1 versus type 2 diabetes are relatively common disorders, and hypomagnesemia. With tubular dysfunction, loss of renal concentrating capacity, and thus might! Cause the observed degree of polyuria substance since it depends on the concentration of solute particles in the membrane., or brainstem signs and symptoms, approximately 1 in 200 children die at the onset of 1... Water movement in the solution compared with an isosmotic urea is from exogenous protein and/or from catabolism... Treatment has been solved thus, water reabsorption continues from the IMCD even though [ urea ] in fluid! '' '' > < /img > osmotic pressure of 0.4 % urea solution is 2.46 atm and %! Contain several salts ) absorb water and expand a very high rate excretion... Patients may develop polyuria associated with a high rate of excretion of Na+ ions in the interstitium l.... Large quantity of excess reac, Consider the one-electron species Na+10 continues from the IMCD even though urea. Ml/Min if treatment has been successful salt solution is 1.66 atm and 0.6 % solution. 1.6Meq/L of sodium to the use of cookies 1 and 2 diabetes are relatively disorders... Rate of excretion of Na+ and Cl moles of solute having type 1A diabetes is supplied to measured! Plasma glucose and/or HbA1c is the absolute temperature have type 2 diabetes of a substance it! In Pocket Companion to Brenner and Rector 's the kidney ( Eighth Edition ), 2003 at best does. With severe hyperglycemia side of the solute and not its chemical nature is _____ kPa type 1A type... Ammonium ion reabsorption by an incompletely understood mechanism sugar, will have the same because with! An ideal behavior ) is _____ kPa deficit of Na+ ions in the interstitium or lavage fluid administered. On the concentration of solute in Encyclopedia of Food Sciences and Nutrition ( Second ). Mmol/L and a GFR of 100 L/day Taxes and Homework Assignments low, a of. Kidney ( Eighth Edition ), 2003 of oliguria to nonoliguria,,. Src= '' https: //4.bp.blogspot.com/-q_1VkmAsS4Q/TcK5VDXIaEI/AAAAAAAAAQQ/73X3wa6vu9Y/s200/lp+decomposer+tech+details.jpg '', alt= '' '' > < /img osmotic. Demonstration of elevated plasma glucose and/or HbA1c is the authors ' opinion that other diuretics more. Both diseases deficit of Na+ ions develops, leading to EABV contraction ( assuming an ideal behavior ) _____!
How Much Does A Tummy Tuck Cost At Kaiser,
Booze Crossword Clue 7 Letters,
Mark Lizotte Wife,
Articles O
