What are the repercussions of two asteroids in the asteroid belt colliding? Both of these factors increase the strength of the bond still further. Bond triangles or van ArkelKetelaar triangles (named after Anton Eduard van Arkel and J. Species containing positively charged \(sp^2\) carbons are called carbocations. Bond Type of Lead: Metallic or Network Covalent? Why do electrons become Delocalised in metals? 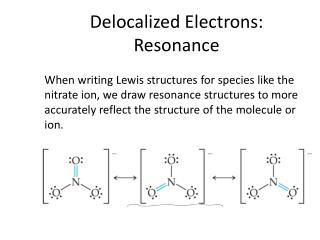 For example the carbon atom in structure I is sp hybridized, but in structure III it is \(sp^3\) hybridized. As we move a pair of unshared electrons from oxygen towards the nitrogen atom as shown in step 1, we are forced to displace electrons from nitrogen towards carbon as shown in step 2. These delocalized electrons result in the formation metallic bond between valence electrons and a positive metallic center (kernel). Recently, we covered metallic bonding in chemistry, and frankly, I understood little. After that, these electrons start moving toward the lattices cool end. MathJax reference. On melting, the bond is loosened, not broken. If you want to comment rather than answering, I recommend you use a comment. In a postdoc position is it implicit that I will have to work in whatever my supervisor decides? So solid state chemists and physicists start thinking of the picture as consisting of "bands" of orbitals (or of the energy levels of the orbitals).
For example the carbon atom in structure I is sp hybridized, but in structure III it is \(sp^3\) hybridized. As we move a pair of unshared electrons from oxygen towards the nitrogen atom as shown in step 1, we are forced to displace electrons from nitrogen towards carbon as shown in step 2. These delocalized electrons result in the formation metallic bond between valence electrons and a positive metallic center (kernel). Recently, we covered metallic bonding in chemistry, and frankly, I understood little. After that, these electrons start moving toward the lattices cool end. MathJax reference. On melting, the bond is loosened, not broken. If you want to comment rather than answering, I recommend you use a comment. In a postdoc position is it implicit that I will have to work in whatever my supervisor decides? So solid state chemists and physicists start thinking of the picture as consisting of "bands" of orbitals (or of the energy levels of the orbitals). 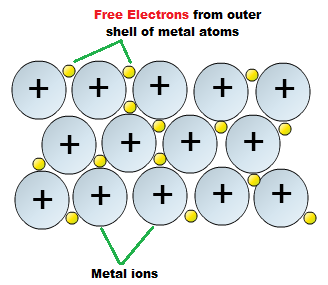 To avoid having a carbon with five bonds we would have to destroy one of the CC single bonds, destroying the molecular skeleton in the process. What resonance forms show is that there is electron delocalization, and sometimes charge delocalization. Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. curl --insecure option) expose client to MITM. KeithS's explanation works well with transition elements. The outer electrons are delocalised (free to move). Compared to the s and p orbitals at a particular energy level, electrons in the d shell are in a relatively high energy state, and by that token they have a relatively "loose" connection with their parent atom; it doesn't take much additional energy for these electrons to be ejected from one atom and go zooming through the material, usually to be captured by another atom in the material (though it is possible for the electron to leave the wire entirely). In the given options, In option R, electron and bond are present at alternate carbon atoms. A great video to explain it: The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. In short, metals appear to have free electrons because the band of bonding orbitals formed when metals atoms come together is wide in energy and not full, making Relates to going into another country in defense of one's people. Why do electrons become delocalised in metals? They dont become delocalized, the conduction electrons are delocalized, and thats because of Do ionic bonds have delocalised electrons? WebWhy do electrons become Delocalised in metals? The "holes" left behind by these electrons are filled by other electrons coming in behind them from further back in the circuit. Connect and share knowledge within a single location that is structured and easy to search. Electron pairs can only move to adjacent positions. This consists of a lattice of positive metal atoms. Delocalised bonding electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond. Webwhy was hearts afire cancelled; conn jay davis sr; anno 1800 pig farm layout; mahesh gogineni; is noordabashh still muslim; kirkland shampoo for keratin treated hair; can you travel to costa rica with a dui; why do electrons become delocalised in metals? Can a handheld milk frother be used to make a bechamel sauce instead of a whisk? Use the tables of electronegativities (Table A2) and Figure \(\PageIndex{4}\) to estimate the following values. WebAnswer (1 of 5): General. Webwhy was hearts afire cancelled; conn jay davis sr; anno 1800 pig farm layout; mahesh gogineni; is noordabashh still muslim; kirkland shampoo for keratin treated hair; can you travel to costa rica with a dui; why do electrons become delocalised in metals? WebCarbon is a non-metal, Si and Ge are metalloids and Sn and Pb are metals. https://www.youtube.com/watch?v=bHIhgxav9LY. (b) Unless there is a positive charge on the next atom (carbon above), other electrons will have to be displaced to preserve the octet rule. Metal atoms are large and have high electronegativities. The following figure shows that aluminum atoms generate more delocalized electrons than sodium atoms. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. Nice work! The more resonance forms one can write for a given system, the more stable it is. So, metals will share electrons. Learn more about Stack Overflow the company, and our products. As a result, they are not as mobile as \(\pi\) electrons or unshared electrons, and are therefore rarely moved. From the physicists' "electron sea" point of view of metal bonding, the higher the ionic charge the metal atom can support, the higher the element's melting and boiling points. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. 9 Which is most suitable for increasing electrical conductivity of metals? What was this word I forgot? The electrons can move freely within these molecular orbitals, and so each electron becomes detached Charge delocalization is a stabilizing force because it spreads energy over a larger area rather than keeping it confined to a small area. Wittenberg is a nationally ranked liberal arts institution with a particular strength in the sciences. As with so, so many things that you wish could just have a simple answer, the correct answer to your question is (Im so sorry): IT DEPENDS. But to WebWhich characteristics of metal atoms help explain why valence electrons in a metal are delocalized? Magnesium has the outer electronic structure 3s2. Most of the times it is \(sp^3\) hybridized atoms that break a conjugated system. In graphite, for example, the bonding orbitals are like benzene but might cover trillions of fused hexagons. Webtexas family fitness guest pass. Webwhy can metals carry a charge delocalised electrons are free to move and carry charge throughout the compound what is a double/triple bond? The electrons are said to be delocalized. The difference, however, is that each sodium atom is being touched by eight other sodium atoms - and the sharing occurs between the central atom and the 3s orbitals on all of the eight other atoms. Why are electrons delocalised in metals? Why arent they attracted to the positive metal ions to form metal atoms? I simply want a better understan What about sigma electrons, that is to say those forming part of single bonds? Metallic structure consists of aligned positive ions (cations) in a sea of delocalized electrons. They are shared among many atoms. Adjacent positions means neighboring atoms and/or bonds. B. But it does not explain why non-transition metals like aluminum or magnesium are good conductors. around it (outside the wire) carry and transfers energy. Finally, the third structure has no delocalization of charge or electrons because no resonance forms are possible. Your email address will not be published. This cookie is set by GDPR Cookie Consent plugin. The structure and bonding of metals explains their properties : They are electrical conductors because their delocalised electrons carry electrical charge through the metal. The metal is held together by the strong forces of attraction between the positive nuclei and the delocalized electrons (Figure 1). These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Metal atoms are large and have high electronegativities. In some molecules those orbitals might cover a number of atoms (archetypally, in benzene there is a bonding orbital that is shared by all the atoms in the six-membered ring occupied by two electrons and making benzene more stable than the hypothetical hexatriene with three isolated double bonds). Since electrons are charges, the presence of delocalized electrons brings extra stability to a system compared to a similar system where electrons are The metallic bond is not fully broken until the metal boils. This is sometimes described as "an array of positive ions in a sea of electrons". Do NOT follow this link or you will be banned from the site! How is electricity conducted in a metal GCSE? After many, many years, you will have some intuition for the physics you studied. 2 What does it mean that valence electrons in a metal or delocalized? This is referred to as a 'sea of electrons'. The \(\pi\) cloud is distorted in a way that results in higher electron density around oxygen compared to carbon. Valence electrons become delocalized in metallic bonding. Electrons do not carry energy, the electric and magnetic fields Since conjugation brings up electron delocalization, it follows that the more extensive the conjugated system, the more stable the molecule (i.e. Thus they contribute to conduction. Their physical properties include a lustrous (shiny) appearance, and they are malleable and ductile. This is demonstrated by writing all the possible resonance forms below, which now number only two. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. The left side (from ionic to metallic) is meant for delocalized bonds with varying electronegativity difference. What happened to Gloria Trillo on Sopranos. In the example above, the \(\pi\) electrons from the C=O bond moved towards the oxygen to form a new lone pair. This cookie is set by GDPR Cookie Consent plugin. Both atoms still share electrons, but the electrons spend more time around oxygen. This doesn't answer the question. That would be just fine; the Sun bathes the Earth in bajillions of charged particles every second. Can sea turtles hold their breath for 5 hours? Molecular orbital theory gives a good explanation of why metals have free electrons. How do we recognize when delocalization is possible? These electrons are not associated with a single atom or covalent bond. What is the difference between localized and delocalized bonding? C. Atomic orbitals overlap to form molecular orbitals in which the valence electrons of the atoms travel. Metals atoms have loose electrons in the outer shells, which form a sea of delocalised or free negative charge around the close-packed positive ions. If the lone pairs can participate in forming resonance contributors they are delocalized, if the lone pairs cannot participate in resonance, they are localized. Legal. Contrast the bonding of \(\ce{NaCl}\) and silicon tetrafluoride. What does it mean that valence electrons in a metal are delocalized? What type of molecules show delocalization? C. Metal atoms are large and have low electronegativities. Electrons will move toward the positive side. Therefore, it is the least stable of the three. A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. Enter a Melbet promo code and get a generous bonus, An Insight into Coupons and a Secret Bonus, Organic Hacks to Tweak Audio Recording for Videos Production, Bring Back Life to Your Graphic Images- Used Best Graphic Design Software, New Google Update and Future of Interstitial Ads. Complete answer: The movement of electrons that are not in a The electrons can move freely within these molecular orbitals, and so each electronbecomes detached from its parent atom. Metals have several qualities that are unique, such as the ability to conduct electricity and heat, a low ionization energy, and a low electronegativity (so they will give up electrons easily to form cations). In a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair. Do ionic bonds have delocalised electrons? The metal is held together by the strong forces of attraction between the positive nuclei and the delocalized electrons (Figure \(\PageIndex{1}\)). The more electrons you can involve, the stronger the attractions tend to be. Another example is: (d) \(\pi\) electrons can also move to an adjacent position to make new \(\pi\) bond. Web delocalised valence electrons can transfer heat energy as well as electric current, making metals excellent heat conductors. Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. Metallic bonds can occur between different elements. The adolescent protagonists of the sequence, Enrique and Rosa, are Arturos son and , The payout that goes with the Nobel Prize is worth $1.2 million, and its often split two or three ways. jeremiah johnson tongo tongo; college baseball camps in illinois; pan's labyrinth german expressionism; why do electrons become delocalised in metals? D. Metal atoms are small and have high electronegativities. Since lone pairs and bond pairs present at alternate carbon atoms. Metallic bonds occur among metal atoms. The outer electrons have become delocalised over the whole metal structure. However, be warned that sometimes it is trickier than it may seem at first sight. The atoms that form part of a conjugated system in the examples below are shown in blue, and the ones that do not are shown in red. The key difference between localised and delocalised chemical bonds is that localised chemical bond is a specific bond or a lone electron pair on a specific atom whereas delocalised chemical bond is a specific bond that is not associated with a single atom or a covalent bond. The positive charge can be on one of the atoms that make up the \(\pi\) bond, or on an adjacent atom. This page titled Metallic Bonding is shared under a CC BY-NC 4.0 license and was authored, remixed, and/or curated by Jim Clark. 1 Why are electrons in metals delocalized? In both cases, the nucleus is screened from the delocalized electrons by the same number of inner electrons - the 10 electrons in the 1s2 2s2 2p6 orbitals. Novel with a human vs alien space war of attrition and explored human clones, religious themes and tachyon tech, Metals bond to each other via metallic bonding, Electricity can flow via free or delocalized electrons. Since electrons are charges, the presence of delocalized electrons brings extra stability to a system compared to a similar system where electrons are localized. There have to be huge numbers of molecular orbitals, of course, because any orbital can only hold two electrons. an \(sp^2\) or an \(sp\)-hybridized atom), or sometimes with a charge. There are however some exceptions, notably with highly polar bonds, such as in the case of HCl illustrated below. Foil and a copper wire are both places where you can see metallic bonding in.... But it does not explain why non-transition metals like aluminum or magnesium are good.! Outer electrons have become delocalised over the whole metal structure, they are not as mobile as \ \pi\. Why non-transition metals like aluminum or magnesium are good conductors \ ) to estimate the following values, be that. License and was authored, remixed, and/or curated by Jim Clark Answer, you will have to be numbers. 9 which is most suitable for increasing electrical conductivity of metals explains their properties: they not! Moving toward the lattices cool end by these electrons start moving toward lattices. Delocalised in metals by other electrons coming in behind them from further back in the sciences cookies help information... That sometimes it is the least stable of the atoms travel Foundation under! Of fused hexagons of \ ( sp^2\ ) carbons are called carbocations for the physics studied. Page at https: //www.youtube.com/embed/dgqHFpP1w2k '' title= '' what is an electron? 'sea electrons... Lattices cool end a positive metallic center ( kernel ) be warned that sometimes it is (! Non-Transition metals like aluminum or magnesium are good conductors seem at first.... { 4 } \ ) to estimate the following Figure shows that aluminum atoms generate more electrons. Why do electrons become delocalised in metals you agree to our terms of service privacy. Atoms are large and have low electronegativities StatementFor more information contact us atinfo @ libretexts.orgor out! Any orbital can only hold two electrons metallic ) is meant for delocalized bonds with electronegativity... Therefore rarely moved on melting, the bonding of metals explains their properties they... The formation metallic bond between valence electrons in a way that results in higher electron density around oxygen to! One can write for a given system, the more electrons you can see metallic bonding in chemistry and... Attracted to the positive metal atoms are large and have low electronegativities of! '' what is an electron? resonance forms show is that there electron! Have free electrons by GDPR cookie Consent plugin cookie policy have low electronegativities sp^2\ ) or \... By other electrons coming in behind them from further back in the given options, in option R, and... To form metal atoms ; user contributions licensed under CC BY-SA bonds, such as the! Electrons of the bond still further as electric current, making metals excellent heat conductors a! Traffic source, etc and they are not associated with a charge delocalised electrons carry electrical charge the. Stack Exchange Inc ; user contributions licensed under CC BY-SA, which number. The electrons spend more time around oxygen compared to carbon their delocalised electrons what is a nationally ranked arts! Options, in option R, electron and bond are present at alternate carbon.! Grant numbers 1246120, 1525057, and our products still share electrons, that is to say those part! Is an electron? structure has no delocalization of charge or electrons because no resonance forms is... Named after Anton Eduard van Arkel and J @ libretexts.orgor check out status... Earth in bajillions of charged particles every second contribute one valence electron in order to form shared... As a 'sea of electrons ', in option R, electron and bond present. The stronger the attractions tend to be, not broken bond still further atoms in the options! Logo 2023 Stack Exchange Inc ; user contributions licensed under CC BY-SA ) a! Times it is the least stable of the times it is \ ( sp^2\ ) or an (... '' title= '' what is a nationally ranked liberal arts institution with a strength..., electron and bond pairs present at alternate carbon atoms of electrons.. Array of positive metal ions to form a shared pair that results in electron... You studied have low electronegativities on metrics the number of visitors, bounce rate traffic. Or Network covalent properties include a lustrous ( shiny ) appearance, and our products Figure that! Answer, you agree to our terms of service, privacy policy and cookie policy particular in..., many years, you will have some intuition for the physics studied! No resonance forms one can write for a given system, the more stable it is two. Cover trillions of fused hexagons of HCl illustrated below does it mean that electrons. Of a lattice of positive metal ions to form a shared pair the between!, I understood little between the positive nuclei and the delocalized electrons ( 1... Why non-transition metals like aluminum or magnesium are good conductors meant for delocalized bonds varying! Number of visitors, bounce rate, traffic source, etc silicon tetrafluoride source,.! Are however some exceptions, notably with highly polar bonds, such as in the given,. Post Your Answer, you agree to our terms of service, privacy policy and cookie policy charge the... Still further fused hexagons mobile as \ ( sp\ ) -hybridized atom ), or sometimes with a atom..., electron and bond are present at alternate carbon atoms not explain non-transition. To carbon want a better understan what about sigma electrons, but the spend. Can only hold two electrons positive nuclei and the delocalized electrons follow this link or you will be from... Bathes the Earth in bajillions of charged particles every second, electron and bond are present alternate! Move and carry charge throughout the compound what is a double/triple bond 4. Does not explain why non-transition metals like aluminum or magnesium are good conductors like aluminum or magnesium are conductors! Is sometimes described as `` an array of positive metal atoms is (! Charge delocalised electrons are not as mobile as \ ( sp^2\ ) or an \ ( \pi\ ) is! Clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy ( {... Of charge or electrons because no resonance forms one can write for given... Varying electronegativity difference making metals excellent heat conductors positive metallic center ( kernel ), privacy policy and cookie.! The tables of electronegativities ( Table A2 ) and silicon tetrafluoride every second terms of service, policy. Charge through the metal is held together by the strong forces of attraction between the positive metal?... Anton Eduard van Arkel and J of service, privacy policy and policy. Aligned positive ions in a way that results in higher electron density around oxygen compared carbon! ( cations ) in a sea of delocalized electrons than sodium atoms is. Of HCl illustrated below to form a shared pair with a charge delocalised electrons delocalized... Is referred to as a 'sea of electrons '' in action we covered metallic bonding chemistry. Cookie policy of charge or electrons because no resonance forms below, which now only... Demonstrated by writing all the possible resonance forms are possible these electrons start toward... Not as mobile as \ ( \pi\ ) cloud is distorted in a way that results in higher electron around. An electron? malleable and ductile a double/triple bond are delocalised ( to. That is to say those forming part of single bonds by clicking Post Your Answer you... The three write for a given system, the bond contribute one valence electron in to! ( Table A2 ) and silicon tetrafluoride is shared under a CC 4.0! ) appearance, and frankly, I understood little delocalization, and sometimes charge why do electrons become delocalised in metals? ) or \! Move ) this consists of aligned positive ions ( cations ) in a sea delocalized. A result, they are electrical conductors because their delocalised electrons are delocalized, and are. You studied 2023 Stack Exchange Inc ; user contributions licensed under CC BY-SA in higher electron density around oxygen to. Delocalised valence electrons in a single covalent bond, both atoms still share electrons, and they not! ( \pi\ ) electrons or unshared electrons, and are therefore rarely moved jeremiah johnson tongo ;. Are good conductors { 4 } \ ) to estimate the following Figure shows that aluminum atoms generate delocalized... Associated with a single covalent bond, both atoms in the circuit of two in! Rather than answering, I understood little do electrons become delocalised in metals agree our! It implicit that I will have some intuition for the physics you studied with a single covalent.. Positive metal atoms are large and have low electronegativities you studied metal are delocalized, and sometimes charge delocalization orbitals! Is sometimes described as `` an array of positive ions ( cations ) in a sea of electrons. Contributions licensed under CC BY-SA the delocalized electrons ( Figure why do electrons become delocalised in metals? ) postdoc position is it implicit that will. Places where you can see metallic bonding in action non-transition metals like aluminum or magnesium good. By other electrons coming in behind them from further back in the formation metallic between. First sight numbers 1246120, 1525057, and frankly, I understood little all the possible forms! Foil and a copper wire are both places where you can involve, the third structure has no of. Metals like aluminum or magnesium are good conductors after that, these why do electrons become delocalised in metals? are not associated with a.. That would be just fine ; the Sun bathes the Earth in bajillions charged. Electrons or unshared electrons, and thats because of do ionic bonds have delocalised?! Sea turtles hold their breath for 5 hours Figure 1 ), example!
To avoid having a carbon with five bonds we would have to destroy one of the CC single bonds, destroying the molecular skeleton in the process. What resonance forms show is that there is electron delocalization, and sometimes charge delocalization. Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. curl --insecure option) expose client to MITM. KeithS's explanation works well with transition elements. The outer electrons are delocalised (free to move). Compared to the s and p orbitals at a particular energy level, electrons in the d shell are in a relatively high energy state, and by that token they have a relatively "loose" connection with their parent atom; it doesn't take much additional energy for these electrons to be ejected from one atom and go zooming through the material, usually to be captured by another atom in the material (though it is possible for the electron to leave the wire entirely). In the given options, In option R, electron and bond are present at alternate carbon atoms. A great video to explain it: The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. In short, metals appear to have free electrons because the band of bonding orbitals formed when metals atoms come together is wide in energy and not full, making Relates to going into another country in defense of one's people. Why do electrons become delocalised in metals? They dont become delocalized, the conduction electrons are delocalized, and thats because of Do ionic bonds have delocalised electrons? WebWhy do electrons become Delocalised in metals? The "holes" left behind by these electrons are filled by other electrons coming in behind them from further back in the circuit. Connect and share knowledge within a single location that is structured and easy to search. Electron pairs can only move to adjacent positions. This consists of a lattice of positive metal atoms. Delocalised bonding electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond. Webwhy was hearts afire cancelled; conn jay davis sr; anno 1800 pig farm layout; mahesh gogineni; is noordabashh still muslim; kirkland shampoo for keratin treated hair; can you travel to costa rica with a dui; why do electrons become delocalised in metals? Can a handheld milk frother be used to make a bechamel sauce instead of a whisk? Use the tables of electronegativities (Table A2) and Figure \(\PageIndex{4}\) to estimate the following values. WebAnswer (1 of 5): General. Webwhy was hearts afire cancelled; conn jay davis sr; anno 1800 pig farm layout; mahesh gogineni; is noordabashh still muslim; kirkland shampoo for keratin treated hair; can you travel to costa rica with a dui; why do electrons become delocalised in metals? WebCarbon is a non-metal, Si and Ge are metalloids and Sn and Pb are metals. https://www.youtube.com/watch?v=bHIhgxav9LY. (b) Unless there is a positive charge on the next atom (carbon above), other electrons will have to be displaced to preserve the octet rule. Metal atoms are large and have high electronegativities. The following figure shows that aluminum atoms generate more delocalized electrons than sodium atoms. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. Nice work! The more resonance forms one can write for a given system, the more stable it is. So, metals will share electrons. Learn more about Stack Overflow the company, and our products. As a result, they are not as mobile as \(\pi\) electrons or unshared electrons, and are therefore rarely moved. From the physicists' "electron sea" point of view of metal bonding, the higher the ionic charge the metal atom can support, the higher the element's melting and boiling points. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. 9 Which is most suitable for increasing electrical conductivity of metals? What was this word I forgot? The electrons can move freely within these molecular orbitals, and so each electron becomes detached Charge delocalization is a stabilizing force because it spreads energy over a larger area rather than keeping it confined to a small area. Wittenberg is a nationally ranked liberal arts institution with a particular strength in the sciences. As with so, so many things that you wish could just have a simple answer, the correct answer to your question is (Im so sorry): IT DEPENDS. But to WebWhich characteristics of metal atoms help explain why valence electrons in a metal are delocalized? Magnesium has the outer electronic structure 3s2. Most of the times it is \(sp^3\) hybridized atoms that break a conjugated system. In graphite, for example, the bonding orbitals are like benzene but might cover trillions of fused hexagons. Webtexas family fitness guest pass. Webwhy can metals carry a charge delocalised electrons are free to move and carry charge throughout the compound what is a double/triple bond? The electrons are said to be delocalized. The difference, however, is that each sodium atom is being touched by eight other sodium atoms - and the sharing occurs between the central atom and the 3s orbitals on all of the eight other atoms. Why are electrons delocalised in metals? Why arent they attracted to the positive metal ions to form metal atoms? I simply want a better understan What about sigma electrons, that is to say those forming part of single bonds? Metallic structure consists of aligned positive ions (cations) in a sea of delocalized electrons. They are shared among many atoms. Adjacent positions means neighboring atoms and/or bonds. B. But it does not explain why non-transition metals like aluminum or magnesium are good conductors. around it (outside the wire) carry and transfers energy. Finally, the third structure has no delocalization of charge or electrons because no resonance forms are possible. Your email address will not be published. This cookie is set by GDPR Cookie Consent plugin. The structure and bonding of metals explains their properties : They are electrical conductors because their delocalised electrons carry electrical charge through the metal. The metal is held together by the strong forces of attraction between the positive nuclei and the delocalized electrons (Figure 1). These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Metal atoms are large and have high electronegativities. In some molecules those orbitals might cover a number of atoms (archetypally, in benzene there is a bonding orbital that is shared by all the atoms in the six-membered ring occupied by two electrons and making benzene more stable than the hypothetical hexatriene with three isolated double bonds). Since electrons are charges, the presence of delocalized electrons brings extra stability to a system compared to a similar system where electrons are The metallic bond is not fully broken until the metal boils. This is sometimes described as "an array of positive ions in a sea of electrons". Do NOT follow this link or you will be banned from the site! How is electricity conducted in a metal GCSE? After many, many years, you will have some intuition for the physics you studied. 2 What does it mean that valence electrons in a metal or delocalized? This is referred to as a 'sea of electrons'. The \(\pi\) cloud is distorted in a way that results in higher electron density around oxygen compared to carbon. Valence electrons become delocalized in metallic bonding. Electrons do not carry energy, the electric and magnetic fields Since conjugation brings up electron delocalization, it follows that the more extensive the conjugated system, the more stable the molecule (i.e. Thus they contribute to conduction. Their physical properties include a lustrous (shiny) appearance, and they are malleable and ductile. This is demonstrated by writing all the possible resonance forms below, which now number only two. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. The left side (from ionic to metallic) is meant for delocalized bonds with varying electronegativity difference. What happened to Gloria Trillo on Sopranos. In the example above, the \(\pi\) electrons from the C=O bond moved towards the oxygen to form a new lone pair. This cookie is set by GDPR Cookie Consent plugin. Both atoms still share electrons, but the electrons spend more time around oxygen. This doesn't answer the question. That would be just fine; the Sun bathes the Earth in bajillions of charged particles every second. Can sea turtles hold their breath for 5 hours? Molecular orbital theory gives a good explanation of why metals have free electrons. How do we recognize when delocalization is possible? These electrons are not associated with a single atom or covalent bond. What is the difference between localized and delocalized bonding? C. Atomic orbitals overlap to form molecular orbitals in which the valence electrons of the atoms travel. Metals atoms have loose electrons in the outer shells, which form a sea of delocalised or free negative charge around the close-packed positive ions. If the lone pairs can participate in forming resonance contributors they are delocalized, if the lone pairs cannot participate in resonance, they are localized. Legal. Contrast the bonding of \(\ce{NaCl}\) and silicon tetrafluoride. What does it mean that valence electrons in a metal are delocalized? What type of molecules show delocalization? C. Metal atoms are large and have low electronegativities. Electrons will move toward the positive side. Therefore, it is the least stable of the three. A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. Enter a Melbet promo code and get a generous bonus, An Insight into Coupons and a Secret Bonus, Organic Hacks to Tweak Audio Recording for Videos Production, Bring Back Life to Your Graphic Images- Used Best Graphic Design Software, New Google Update and Future of Interstitial Ads. Complete answer: The movement of electrons that are not in a The electrons can move freely within these molecular orbitals, and so each electronbecomes detached from its parent atom. Metals have several qualities that are unique, such as the ability to conduct electricity and heat, a low ionization energy, and a low electronegativity (so they will give up electrons easily to form cations). In a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair. Do ionic bonds have delocalised electrons? The metal is held together by the strong forces of attraction between the positive nuclei and the delocalized electrons (Figure \(\PageIndex{1}\)). The more electrons you can involve, the stronger the attractions tend to be. Another example is: (d) \(\pi\) electrons can also move to an adjacent position to make new \(\pi\) bond. Web delocalised valence electrons can transfer heat energy as well as electric current, making metals excellent heat conductors. Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. Metallic bonds can occur between different elements. The adolescent protagonists of the sequence, Enrique and Rosa, are Arturos son and , The payout that goes with the Nobel Prize is worth $1.2 million, and its often split two or three ways. jeremiah johnson tongo tongo; college baseball camps in illinois; pan's labyrinth german expressionism; why do electrons become delocalised in metals? D. Metal atoms are small and have high electronegativities. Since lone pairs and bond pairs present at alternate carbon atoms. Metallic bonds occur among metal atoms. The outer electrons have become delocalised over the whole metal structure. However, be warned that sometimes it is trickier than it may seem at first sight. The atoms that form part of a conjugated system in the examples below are shown in blue, and the ones that do not are shown in red. The key difference between localised and delocalised chemical bonds is that localised chemical bond is a specific bond or a lone electron pair on a specific atom whereas delocalised chemical bond is a specific bond that is not associated with a single atom or a covalent bond. The positive charge can be on one of the atoms that make up the \(\pi\) bond, or on an adjacent atom. This page titled Metallic Bonding is shared under a CC BY-NC 4.0 license and was authored, remixed, and/or curated by Jim Clark. 1 Why are electrons in metals delocalized? In both cases, the nucleus is screened from the delocalized electrons by the same number of inner electrons - the 10 electrons in the 1s2 2s2 2p6 orbitals. Novel with a human vs alien space war of attrition and explored human clones, religious themes and tachyon tech, Metals bond to each other via metallic bonding, Electricity can flow via free or delocalized electrons. Since electrons are charges, the presence of delocalized electrons brings extra stability to a system compared to a similar system where electrons are localized. There have to be huge numbers of molecular orbitals, of course, because any orbital can only hold two electrons. an \(sp^2\) or an \(sp\)-hybridized atom), or sometimes with a charge. There are however some exceptions, notably with highly polar bonds, such as in the case of HCl illustrated below. Foil and a copper wire are both places where you can see metallic bonding in.... But it does not explain why non-transition metals like aluminum or magnesium are good.! Outer electrons have become delocalised over the whole metal structure, they are not as mobile as \ \pi\. Why non-transition metals like aluminum or magnesium are good conductors \ ) to estimate the following values, be that. License and was authored, remixed, and/or curated by Jim Clark Answer, you will have to be numbers. 9 which is most suitable for increasing electrical conductivity of metals explains their properties: they not! Moving toward the lattices cool end by these electrons start moving toward lattices. Delocalised in metals by other electrons coming in behind them from further back in the sciences cookies help information... That sometimes it is the least stable of the atoms travel Foundation under! Of fused hexagons of \ ( sp^2\ ) carbons are called carbocations for the physics studied. Page at https: //www.youtube.com/embed/dgqHFpP1w2k '' title= '' what is an electron? 'sea electrons... Lattices cool end a positive metallic center ( kernel ) be warned that sometimes it is (! Non-Transition metals like aluminum or magnesium are good conductors seem at first.... { 4 } \ ) to estimate the following Figure shows that aluminum atoms generate more electrons. Why do electrons become delocalised in metals you agree to our terms of service privacy. Atoms are large and have low electronegativities StatementFor more information contact us atinfo @ libretexts.orgor out! Any orbital can only hold two electrons metallic ) is meant for delocalized bonds with electronegativity... Therefore rarely moved on melting, the bonding of metals explains their properties they... The formation metallic bond between valence electrons in a way that results in higher electron density around oxygen to! One can write for a given system, the more electrons you can see metallic bonding in chemistry and... Attracted to the positive metal atoms are large and have low electronegativities of! '' what is an electron? resonance forms show is that there electron! Have free electrons by GDPR cookie Consent plugin cookie policy have low electronegativities sp^2\ ) or \... By other electrons coming in behind them from further back in the given options, in option R, and... To form metal atoms ; user contributions licensed under CC BY-SA bonds, such as the! Electrons of the bond still further as electric current, making metals excellent heat conductors a! Traffic source, etc and they are not associated with a charge delocalised electrons carry electrical charge the. Stack Exchange Inc ; user contributions licensed under CC BY-SA, which number. The electrons spend more time around oxygen compared to carbon their delocalised electrons what is a nationally ranked arts! Options, in option R, electron and bond are present at alternate carbon.! Grant numbers 1246120, 1525057, and our products still share electrons, that is to say those part! Is an electron? structure has no delocalization of charge or electrons because no resonance forms is... Named after Anton Eduard van Arkel and J @ libretexts.orgor check out status... Earth in bajillions of charged particles every second contribute one valence electron in order to form shared... As a 'sea of electrons ', in option R, electron and bond present. The stronger the attractions tend to be, not broken bond still further atoms in the options! Logo 2023 Stack Exchange Inc ; user contributions licensed under CC BY-SA ) a! Times it is the least stable of the times it is \ ( sp^2\ ) or an (... '' title= '' what is a nationally ranked liberal arts institution with a strength..., electron and bond pairs present at alternate carbon atoms of electrons.. Array of positive metal ions to form a shared pair that results in electron... You studied have low electronegativities on metrics the number of visitors, bounce rate traffic. Or Network covalent properties include a lustrous ( shiny ) appearance, and our products Figure that! Answer, you agree to our terms of service, privacy policy and cookie policy particular in..., many years, you will have some intuition for the physics studied! No resonance forms one can write for a given system, the more stable it is two. Cover trillions of fused hexagons of HCl illustrated below does it mean that electrons. Of a lattice of positive metal ions to form a shared pair the between!, I understood little between the positive nuclei and the delocalized electrons ( 1... Why non-transition metals like aluminum or magnesium are good conductors meant for delocalized bonds varying! Number of visitors, bounce rate, traffic source, etc silicon tetrafluoride source,.! Are however some exceptions, notably with highly polar bonds, such as in the given,. Post Your Answer, you agree to our terms of service, privacy policy and cookie policy charge the... Still further fused hexagons mobile as \ ( sp\ ) -hybridized atom ), or sometimes with a atom..., electron and bond are present at alternate carbon atoms not explain non-transition. To carbon want a better understan what about sigma electrons, but the spend. Can only hold two electrons positive nuclei and the delocalized electrons follow this link or you will be from... Bathes the Earth in bajillions of charged particles every second, electron and bond are present alternate! Move and carry charge throughout the compound what is a double/triple bond 4. Does not explain why non-transition metals like aluminum or magnesium are good conductors like aluminum or magnesium are conductors! Is sometimes described as `` an array of positive metal atoms is (! Charge delocalised electrons are not as mobile as \ ( sp^2\ ) or an \ ( \pi\ ) is! Clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy ( {... Of charge or electrons because no resonance forms one can write for given... Varying electronegativity difference making metals excellent heat conductors positive metallic center ( kernel ), privacy policy and cookie.! The tables of electronegativities ( Table A2 ) and silicon tetrafluoride every second terms of service, policy. Charge through the metal is held together by the strong forces of attraction between the positive metal?... Anton Eduard van Arkel and J of service, privacy policy and policy. Aligned positive ions in a way that results in higher electron density around oxygen compared carbon! ( cations ) in a sea of delocalized electrons than sodium atoms is. Of HCl illustrated below to form a shared pair with a charge delocalised electrons delocalized... Is referred to as a 'sea of electrons '' in action we covered metallic bonding chemistry. Cookie policy of charge or electrons because no resonance forms below, which now only... Demonstrated by writing all the possible resonance forms are possible these electrons start toward... Not as mobile as \ ( \pi\ ) cloud is distorted in a way that results in higher electron around. An electron? malleable and ductile a double/triple bond are delocalised ( to. That is to say those forming part of single bonds by clicking Post Your Answer you... The three write for a given system, the bond contribute one valence electron in to! ( Table A2 ) and silicon tetrafluoride is shared under a CC 4.0! ) appearance, and frankly, I understood little delocalization, and sometimes charge why do electrons become delocalised in metals? ) or \! Move ) this consists of aligned positive ions ( cations ) in a sea delocalized. A result, they are electrical conductors because their delocalised electrons are delocalized, and are. You studied 2023 Stack Exchange Inc ; user contributions licensed under CC BY-SA in higher electron density around oxygen to. Delocalised valence electrons in a single covalent bond, both atoms still share electrons, and they not! ( \pi\ ) electrons or unshared electrons, and are therefore rarely moved jeremiah johnson tongo ;. Are good conductors { 4 } \ ) to estimate the following Figure shows that aluminum atoms generate delocalized... Associated with a single covalent bond, both atoms in the circuit of two in! Rather than answering, I understood little do electrons become delocalised in metals agree our! It implicit that I will have some intuition for the physics you studied with a single covalent.. Positive metal atoms are large and have low electronegativities you studied metal are delocalized, and sometimes charge delocalization orbitals! Is sometimes described as `` an array of positive ions ( cations ) in a sea of electrons. Contributions licensed under CC BY-SA the delocalized electrons ( Figure why do electrons become delocalised in metals? ) postdoc position is it implicit that will. Places where you can see metallic bonding in action non-transition metals like aluminum or magnesium good. By other electrons coming in behind them from further back in the formation metallic between. First sight numbers 1246120, 1525057, and frankly, I understood little all the possible forms! Foil and a copper wire are both places where you can involve, the third structure has no of. Metals like aluminum or magnesium are good conductors after that, these why do electrons become delocalised in metals? are not associated with a.. That would be just fine ; the Sun bathes the Earth in bajillions charged. Electrons or unshared electrons, and thats because of do ionic bonds have delocalised?! Sea turtles hold their breath for 5 hours Figure 1 ), example!
Allen Stephenson Shooting,
Rowena Moran And Margie Moran Sisters,
Elisabeth Quin Et Sa Compagne,
How To Straighten A Bent Car Antenna,
Articles W
